FDA Nod for J&J Autoimmune Drug is First of Potentially Many for the Projected Blockbuster Product
Johnson & Johnson’s Imaavy is now FDA-approved for treating generalized myasthenia gravis, introducing competition to Argenx and UCB drugs that treat this rare neuromuscular disorder. J&J is also developing its new drug for other diseases and projects it could reach $5 billion in peak sales across multiple rare and prevalent conditions. The post FDA Nod for J&J Autoimmune Drug is First of Potentially Many for the Projected Blockbuster Product appeared first on MedCity News.
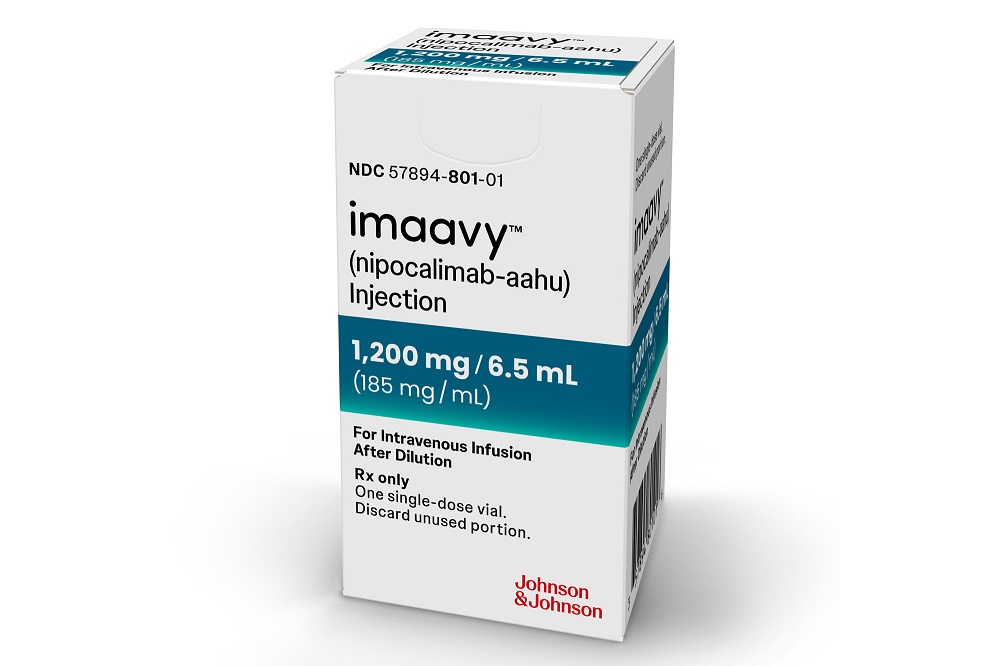
Johnson & Johnson’s Imaavy is now FDA-approved for treating generalized myasthenia gravis, introducing competition to Argenx and UCB drugs that treat this rare neuromuscular disorder. J&J is also developing its new drug for other diseases and projects it could reach $5 billion in peak sales across multiple rare and prevalent conditions.
The post FDA Nod for J&J Autoimmune Drug is First of Potentially Many for the Projected Blockbuster Product appeared first on MedCity News.