Alnylam Drug Approval Brings Cardiomyopathy Competition to Pfizer, BridgeBio Pharma
Amvuttra, an Alnylam drug approved to treat nerve pain from transthyretin amyloidosis, is now FDA-approved for treating cardiomyopathy caused by this disease. The approval brings Amvuttra to a much bigger market, but it must compete with a blockbuster Pfizer drug already established as the standard of care. The post Alnylam Drug Approval Brings Cardiomyopathy Competition to Pfizer, BridgeBio Pharma appeared first on MedCity News.
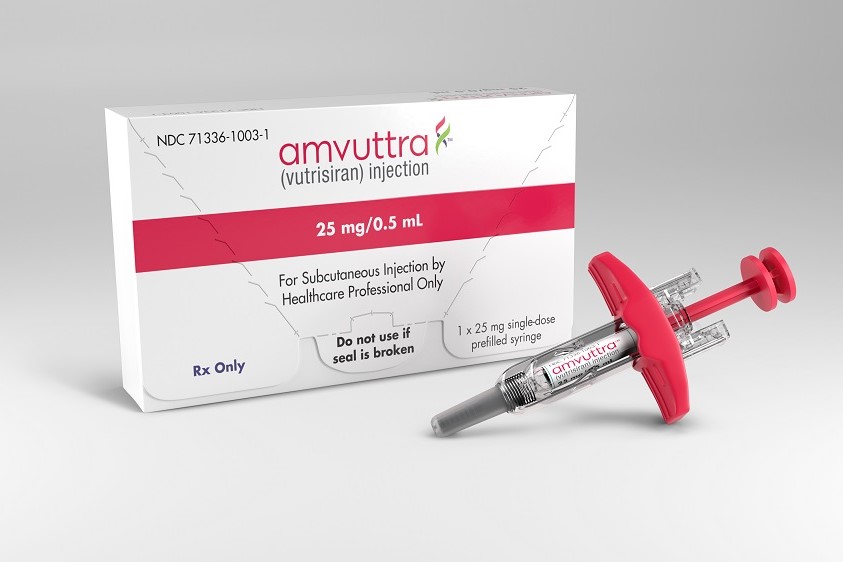
Amvuttra, an Alnylam drug approved to treat nerve pain from transthyretin amyloidosis, is now FDA-approved for treating cardiomyopathy caused by this disease. The approval brings Amvuttra to a much bigger market, but it must compete with a blockbuster Pfizer drug already established as the standard of care.
The post Alnylam Drug Approval Brings Cardiomyopathy Competition to Pfizer, BridgeBio Pharma appeared first on MedCity News.