The science behind the smell of rain
A story of bacteria, arthropods, and two very smelly organic compounds. The post The science behind the smell of rain appeared first on Popular Science.

You know the smell. It’s there every time the first fat raindrops hit the ground—a distinctive, earthy scent that suffuses the air, an aroma that speaks of the changing seasons and promises relief from stifling summer heat. There’s a name for the smell of rain, too: “petrichor,” a poetic portmanteau of the Greek words “petros” (stone) and “ichor” (the blood of the gods in Greek mythology).
Petrichor: the smell of rain. But what causes it?
The name “petrichor” was coined by Australian scientists Isabel Bear and Dick Thomas in 1964, in a paper that constituted perhaps the first serious scientific attempt to explain the phenomenon. The duo used the word to refer to an oil that they distilled from samples of soil and vegetation that were left for up to a year exposed to air and daylight but shielded from rain. They found that the oil contained a complex mixture of volatile organic compounds.
One question left unanswered by Bear and Thomas was the origin of these compounds, and subsequent research has focused on one particular compound, a volatile bicyclic alcohol called geosmin. The compound was isolated a year after Bear and Thomas’s paper, and its name literally means “earth smell.” Along with another volatile organic compound called 2-methylisoborneol or 2-MIB, geosmin is primarily responsible for the characteristic smell of earth—and both contribute greatly to the smell of rain.
Ryan Busby, an ecologist at the US Army’s Corps of Engineers, tells Popular Science that these compounds exist in soil the world over, and that they’re spritzed into the air whenever soil is disturbed.
“[The compounds] accumulate in the pore spaces in the soil,” Busby explains. “There might be some binding to soil particles. [And] research has shown that that impact with the soil surface causes the volatiles to be released into the atmosphere.”
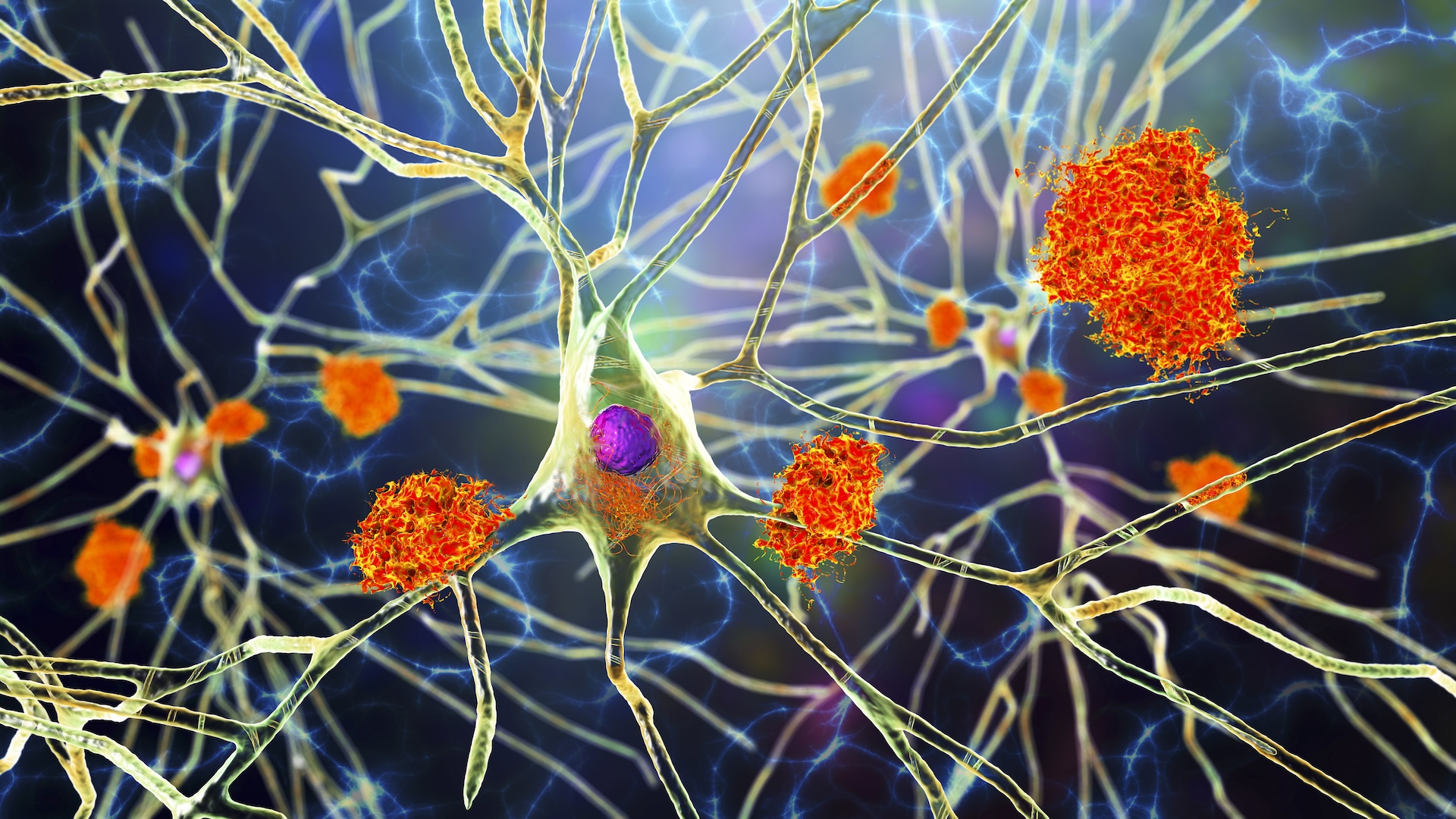
So where do geosmin and 2-MIB come from? Busby says that while the source of both compounds remains the subject of plenty of active research, the current scientific consensus is that they are released by soil-dwelling bacteria.
Differing ratios of the two compounds may explain why the smell differs subtly from place to place.
“Geosmin is pretty consistent across the environment, while 2-MIB is more variable. [Where 2-MIB is present], it is released in much higher concentrations, so you get areas where there’s huge concentrations, and then areas where there’s none,” Busby says. The other components that make up petrichor—a myriad less powerful plant-related volatiles, and also perhaps the distinctive acrid smell of ozone that accompanies lightning—vary from location to location.
Humans are remarkably sensitive to the smell of geosmin, in particular. In water, it can be detected at concentrations as low as 4 ng/L, which equates to about one teaspoon in 200 Olympic swimming pools. Busby says there are several theories for why this might be.

“One [theory] is finding water sources,” he explains. “Geosmin seems to be more prevalent in moist, fertile soils.” The presence of moist soil means the presence of water, and it’s easy to see how being able to catch a whiff of geosmin on the wind and follow it to a source of water would provide a valuable evolutionary advantage.
It’s not just humans who appear to be able to rely on the scent of these volatile compounds to find water, Busby says. “Camels can detect geosmin and find oases in the desert from 50 miles away. Mosquitoes use it to find stagnant ponds for laying eggs, and raccoons use it to find turtle nests and buried eggs.”
But while the smell of geosmin and 2-MIB are appealing to us, their taste is the complete opposite. “It’s kind of funny,” muses Busby. “We love the smell, but we hate the taste.” In water, these compounds are responsible for the musty, moldy taste that indicates that water isn’t safe to drink. Busby says, “Any time you drink water and you think, ‘Oh, this, this tastes like lake water,’ it’s because those compounds are dissolved in what you’re drinking.”
Again, there’s most likely an evolutionary reason for this: it’s one thing for the soil around a water source to smell of bacteria, but if the water itself carries the distinctive musty odor of geosmin and 2-MIB, it also most likely carries the potential for gastrointestinal unpleasantness. Busby says that this explains why geosmin and 2-MIB are “the primary odor contaminants of drinking water globally.”
There’s one unanswered question here, though: why are geosmin and 2-MIB there in the first place? As Busby points out, while it’s clear that “there are a number of uses for geosmin for us, we’re not sure exactly why [bacteria] produce it in such quantities. It’s a [large] energy cost to produce a chemical like that.” So why do soil-borne bacteria pump out geosmin and 2-MIB? What’s in it for them?
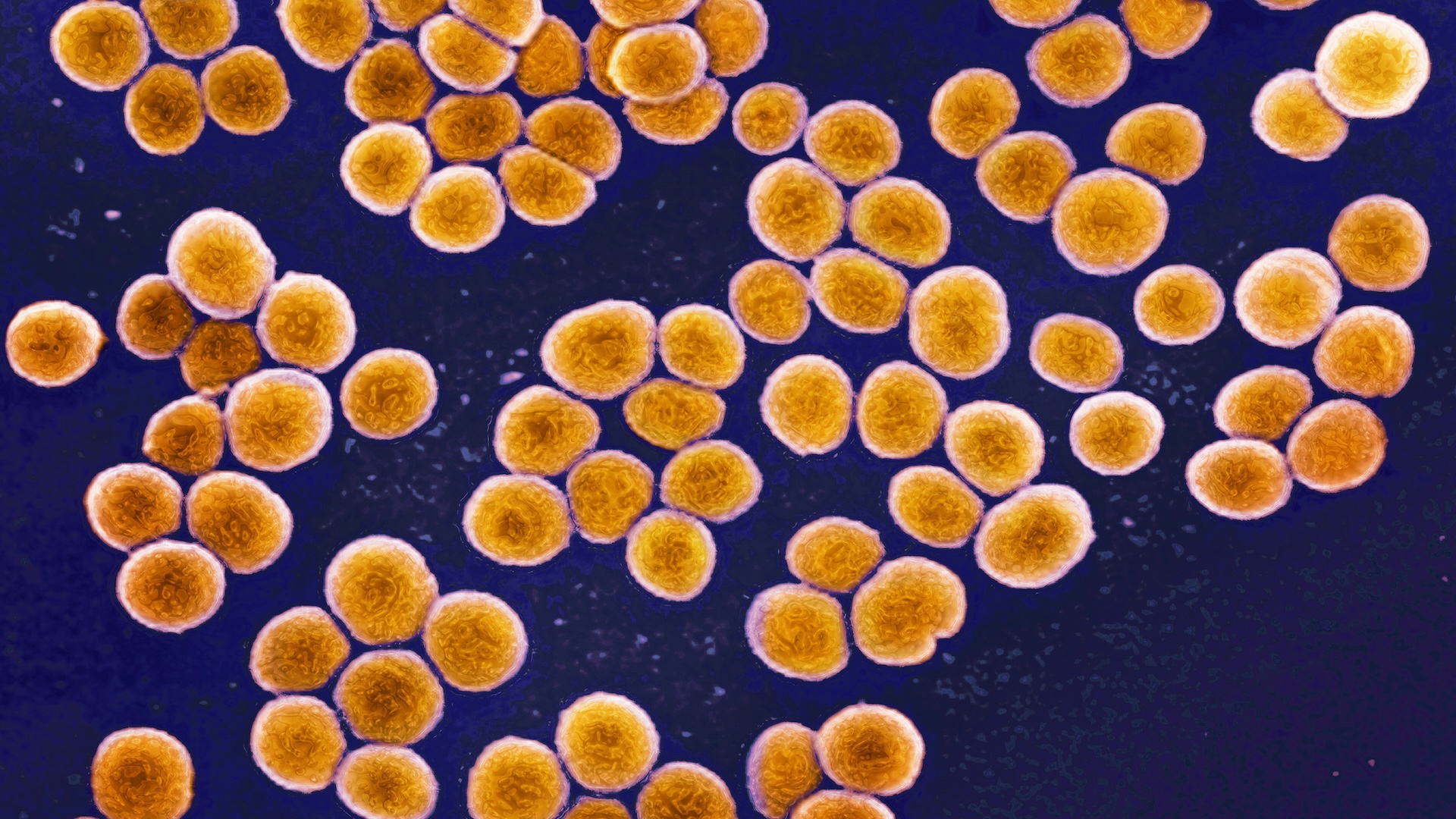
A paper published in Nature Microbiology in 2020 suggested a possible answer. The study examined interactions between Streptomyces—one variety of geosmin- and 2-MIB-producing bacteria—and small creatures called springtails. (Springtails are one of three varieties of six-legged arthropods that are not considered insects, and they have a taste for bacteria.) Crucially, the researchers found that in the bacteria studied, geosmin and 2-MIB were produced only by colonies that were also producing reproductive spores. In fact, they can only be produced by those specific colonies: “The genes for geosmin and 2-MIB synthases are under the direct control of sporulation-specific transcription factors, constraining emission of the odorants to sporulating colonies,” the paper explains.

Springtails are attracted by geosmin and 2-MIB, so unsurprisingly, upon arrival at the odor-emitting colonies, they helped themselves happily to a tasty microbial snack. In doing so, they also consumed the bacterial spores. The spores were then able to pass through the springtail’s digestive tracts and emerge ready for action from the other end.
Busby says this might also explain why the smell of rain is strongest when it comes from rain hitting dry soil. “As soil dries out, the bacteria are going to go dormant, and there seems to be a flush of release [at that point]. So from that respect, [the compounds] are a way to attract something that maybe will carry [the bacteria] to a more conducive environment for growth.”
It might feel like the poetic appeal of petrichor is diminished somewhat by discovering that the oh-so-evocative smell of rain most likely exists to encourage a bunch of tiny arthropods to poop out bacterial spores. But ultimately, it’s another example of nature finding a way—a co-evolutionary relationship that recalls bees and pollen, and one that extends its benefits to the rest of us.
So the next time the rain hits dry soil, think about the tiny bacteria that both lead us to water and stop us drinking from sources that might harm us.
This story is part of Popular Science’s Ask Us Anything series, where we answer your most outlandish, mind-burning questions, from the ordinary to the off-the-wall. Have something you’ve always wanted to know? Ask us.
The post The science behind the smell of rain appeared first on Popular Science.