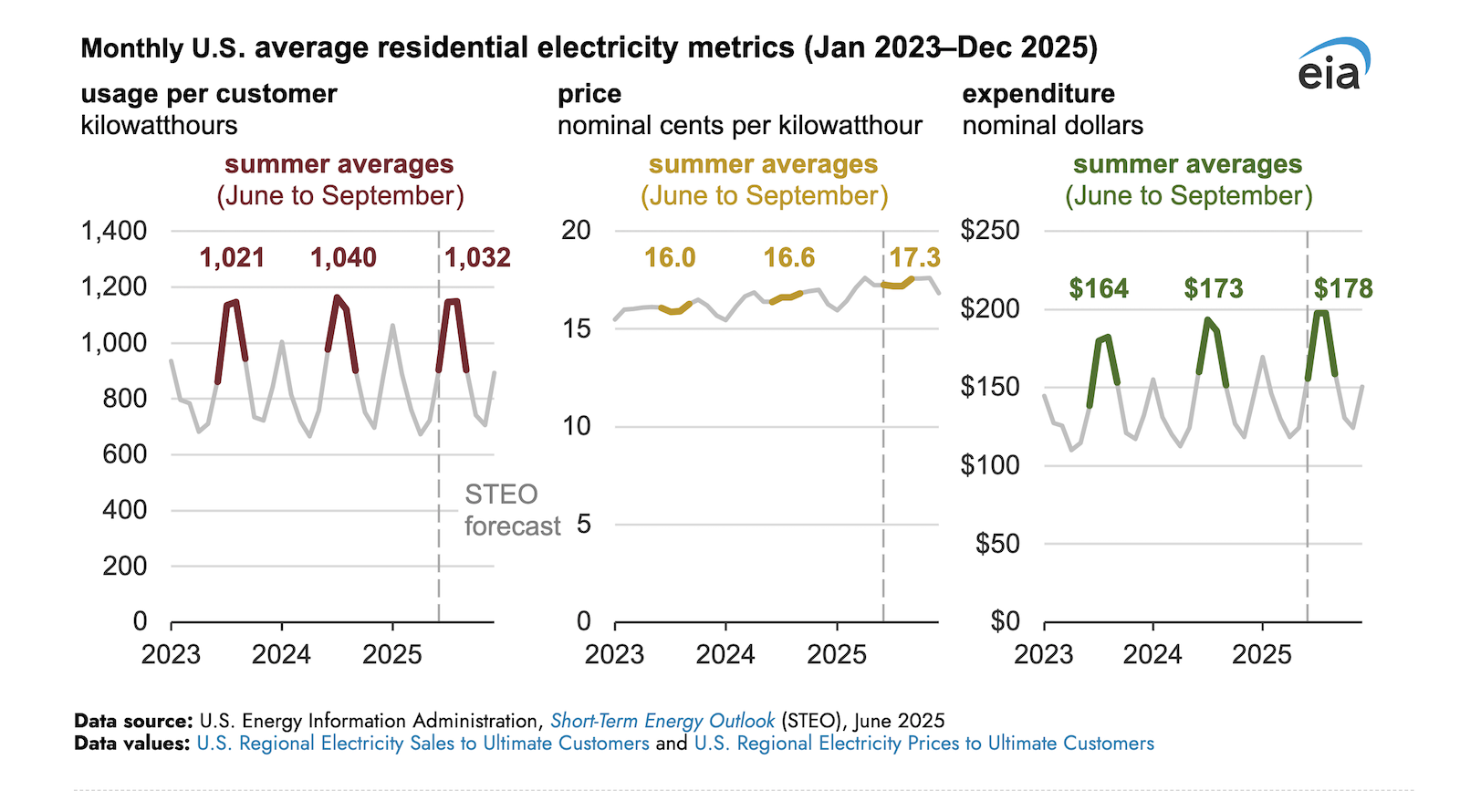
Nuvalent closes in on FDA filing for lung cancer drug with topline Phase 1/2 data
Nuvalent has shared positive data for its ROS1 inhibitor from a “pivotal” mid-stage lung cancer study, and plans to start a rolling NDA submission next month. The biotech’s candidate, called zidesamtinib, achieved a 44% objective ...