Team gets the most detailed image yet of a bacteria-killing virus
Researchers have produced the most detailed image to date of a bacteriophage, or phage—a kind of virus that kills bacteria.
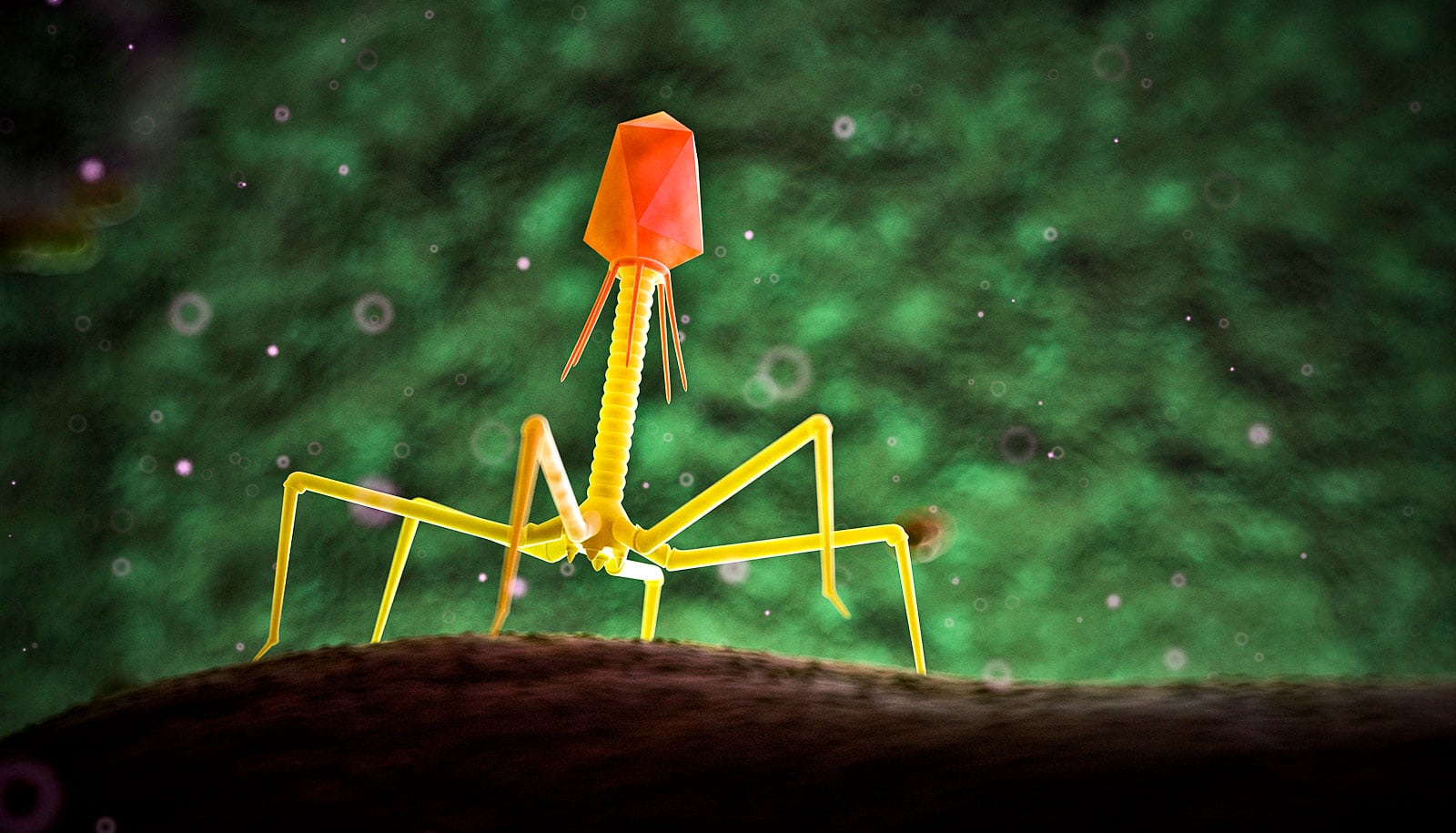

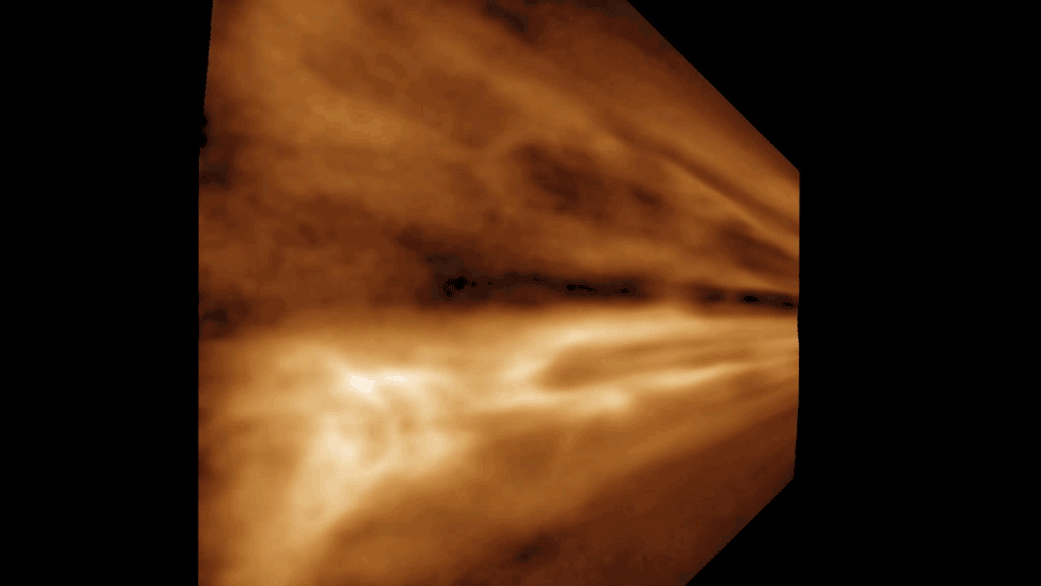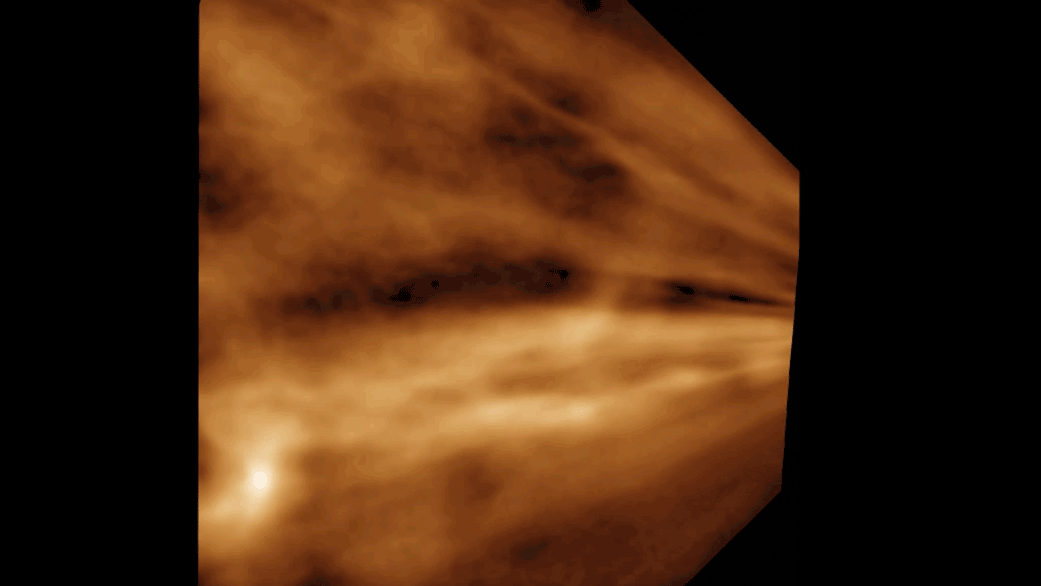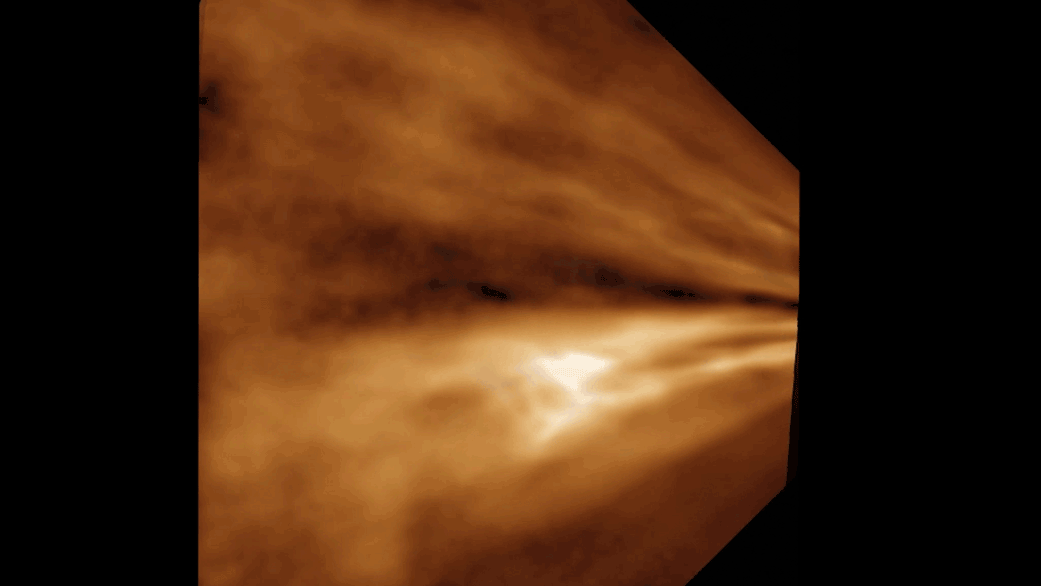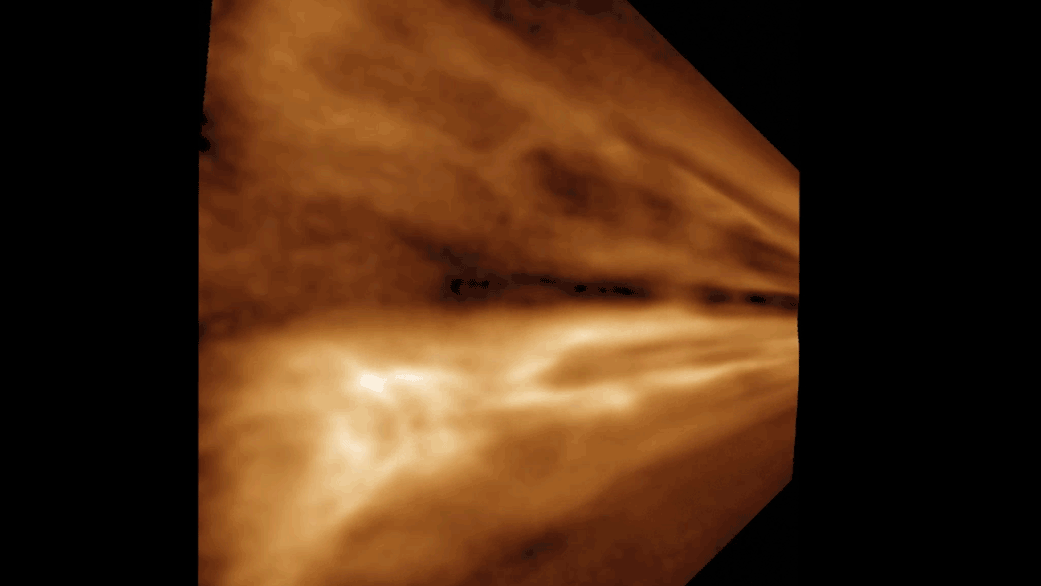
Researchers have produced the most detailed image to date of a bacteriophage, or phage—a kind of virus that kills bacteria—that has allowed them to see for the first time the structural makeup of the part of the virus that directly attaches to its target Mycobacterium cell.
The work could enable new therapies that use bioengineered phages.
“Now you’ve got like a spec sheet for going in and designing phages so that they’ll bind to different kinds of cells,” says Graham Hatfull, a professor of biotechnology at the University of Pittsburgh.
That’s important because of what a bacteriophage, or phage for short, does after it binds to a bacterial cell: it pierces a hole in the cell membrane and injects its own DNA, turning the bacteria into a phage factory.
With many bacteria growing increasingly resistant to the antibiotics we use to kill them, phages are in some cases the only option to fight off bacterial infections. They are, however, picky assassins: A particular kind of phage will typically attack just one strain of bacteria. The ability to engineer a phage to seek out and destroy a specific bacteria could be a medical game-changer.
The research was published in the journal Cell.
Phages have been evolving for billions of years. Despite all the variation this long history has given rise to, they almost all share similar components: a capsid, a tail tube, and a tail tip. Some parts are easier to image than others.
A phage capsid sits like a head atop the narrow tail tube. Researchers have for some time been able to capture high-resolution imagery a phage’s capsid.
“First, it’s gigantic and easy to find,” says Krista Freeman, a research associate in Hatfull’s lab. It’s also composed of 60 symmetrical parts that can be averaged together to boost the signal. Imaging a phage using cryo-electron microscopy, one of the two imaging techniques used in this work, entails stitching together many images from different angles. Because of the part’s symmetry, relatively few images are needed to assemble enough information to piece together an entire capsid.
The rest of the phage’s body is smaller and less symmetric by comparison.
“So you have to be more careful,” Freeman says. “You have to find more particles, and do more hunting, and do more manual manipulations. It’s much less automated than getting the big structure.”
Phages are made up of bundles of tangled, intertwined proteins that loop around and through the structure. They are less like a globe, with its information painted on the surface, and more like a sculpture of a flower—constructed out of Slinkies. In practice, this means piecing together a complete image takes tens of thousands of images of phages, all oriented in different ways.
With this massive amount of data and a massive amount of computing power, Freeman was able to recreate the tail tube and, perhaps most tantalizingly, the tip of the tail, which binds to the bacteria. Currently, researchers do not know why a particular phage attacks a particular strain of bacteria.
“The tip of the tail, that’s the part that’s recognizing the bacteria cell,” Hatfull says. “We’re especially interested in it for this reason.”
Their high-definition images have allowed them to see structures that had previously only been resolved to a fuzzy grayscale that indicated the density of electrons.
“Now you can show every molecular component in this thing,” Freeman says. “And it’s just breathtaking.” The new images also reveal structural information that researchers can explore to better understand the point of contact between a phage and its bacterial target.
“It was astonishing to find out how complex the tail tip structure is,” she says.
The images are made dynamic thanks to work done by Raphael Park and his colleagues at Scripps Research, who used another kind of imaging, cryo-electron tomography, which images phages bound to the bacterial cell, highlighting the entire phage and where it attaches to the bacterial cell surface.
In some images, the phage’s DNA is plainly visible inside the capsid; in others, it has made its way through the cell wall of the bacteria. Between these two steps, “there are some subtle differences,” in the structure of the phage, Hatfull says. These may point to the mechanism by which the DNA is triggered to leave the capsid or how it is transported through the tail tube.
“These are new insights,” Hatfull says. “There are a lot of questions remaining.” But Hatfull, Freeman, and researchers across the world can now start thinking seriously about beginning to engineer phages to recognize different bacteria.
“Before, we wouldn’t have stood a chance. And now, doing this is going to become completely routine.”
The work received support from the National Institute Of General Medical Sciences of the National Institutes of Health and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Source: University of Pittsburgh
The post Team gets the most detailed image yet of a bacteria-killing virus appeared first on Futurity.