Femtech: Overture Life Secures $20.6M to Advance IVF Automation
What You Should Know: – Overture Life today announced a significant $20.6M strategic funding round, bringing its total capital raised to $57M from leading industry investors. – Supported by Overwater Ventures, GV (formerly Google Ventures), and Khosla Ventures, the $20.6M strategic round reflects deep investor conviction in Overture’s science-led mission. – The capital will support ... Read More


What You Should Know:
– Overture Life today announced a significant $20.6M strategic funding round, bringing its total capital raised to $57M from leading industry investors.
– Supported by Overwater Ventures, GV (formerly Google Ventures), and Khosla Ventures, the $20.6M strategic round reflects deep investor conviction in Overture’s science-led mission.
– The capital will support additional clinical research and product development, accelerating the refinement and rollout of technologies like DaVitri .
.
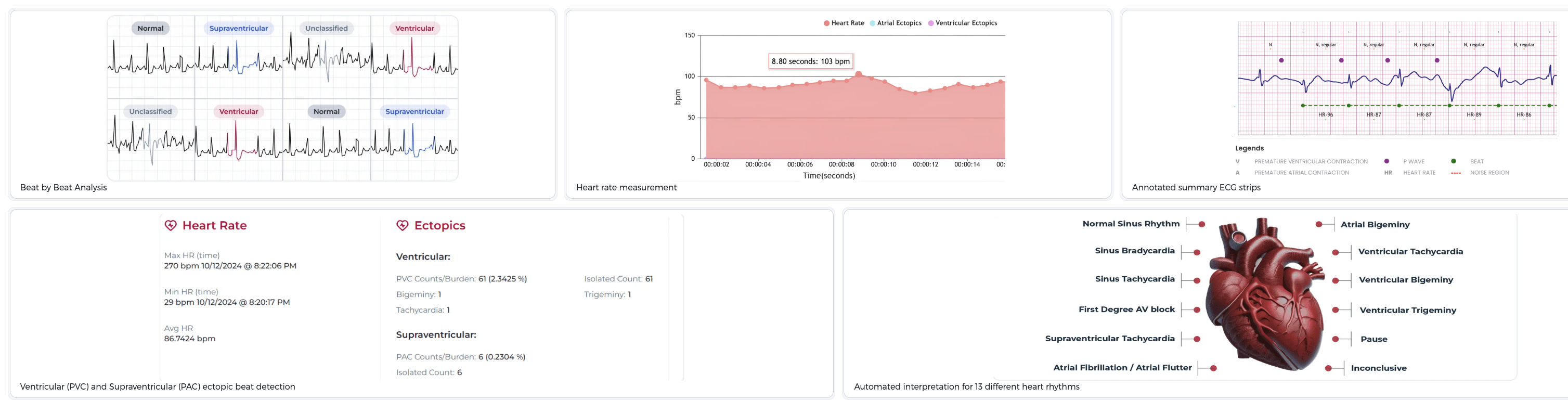
Automating Key Lab Steps to Address IVF Inefficiencies
For too long, high costs and lab inefficiencies have limited access to IVF. Overture Life has been working since 2017 to modernize core IVF technology using proprietary software, robotics, and microfluidics. Its upcoming DaVitri platform specifically targets essential, historically manual lab procedures: egg freezing (vitrification) and embryo warming. By automating these steps with reproducible, data-driven protocols that ensure precise timing and controlled cryoprotectant exposure, DaVitri
platform specifically targets essential, historically manual lab procedures: egg freezing (vitrification) and embryo warming. By automating these steps with reproducible, data-driven protocols that ensure precise timing and controlled cryoprotectant exposure, DaVitri aims to standardize critical processes. Early clinical evaluations suggest the platform can improve oocyte survival rates, potentially reducing the need for repeated IVF cycles that drain patient resources and emotional energy. Its streamlined, gentler freezing process is also designed to help clinics maintain consistent lab results and increase throughput, enabling embryologists to handle more cycles without compromising quality.
aims to standardize critical processes. Early clinical evaluations suggest the platform can improve oocyte survival rates, potentially reducing the need for repeated IVF cycles that drain patient resources and emotional energy. Its streamlined, gentler freezing process is also designed to help clinics maintain consistent lab results and increase throughput, enabling embryologists to handle more cycles without compromising quality.
Commitment to Clinical Rigor and Safety
The new CLIA certification for Overture’s m|z test validates that the company’s U.S. laboratory operations meet stringent federal standards required for clinical diagnostics. This achievement for the AI-powered embryo selection test underscores Overture’s dedication to prioritizing patient safety, securing regulatory support, and demonstrating rigorous scientific validation.
test validates that the company’s U.S. laboratory operations meet stringent federal standards required for clinical diagnostics. This achievement for the AI-powered embryo selection test underscores Overture’s dedication to prioritizing patient safety, securing regulatory support, and demonstrating rigorous scientific validation.
“This new funding allows us to accelerate our expansion of IVF capabilities and demonstrates the value of operating within a CLIA-licensed laboratory environment, highlighting not only our technological leadership but also our commitment to the highest standards of clinical validation,” stated Hans Gangeskar, CEO of Overture Life.