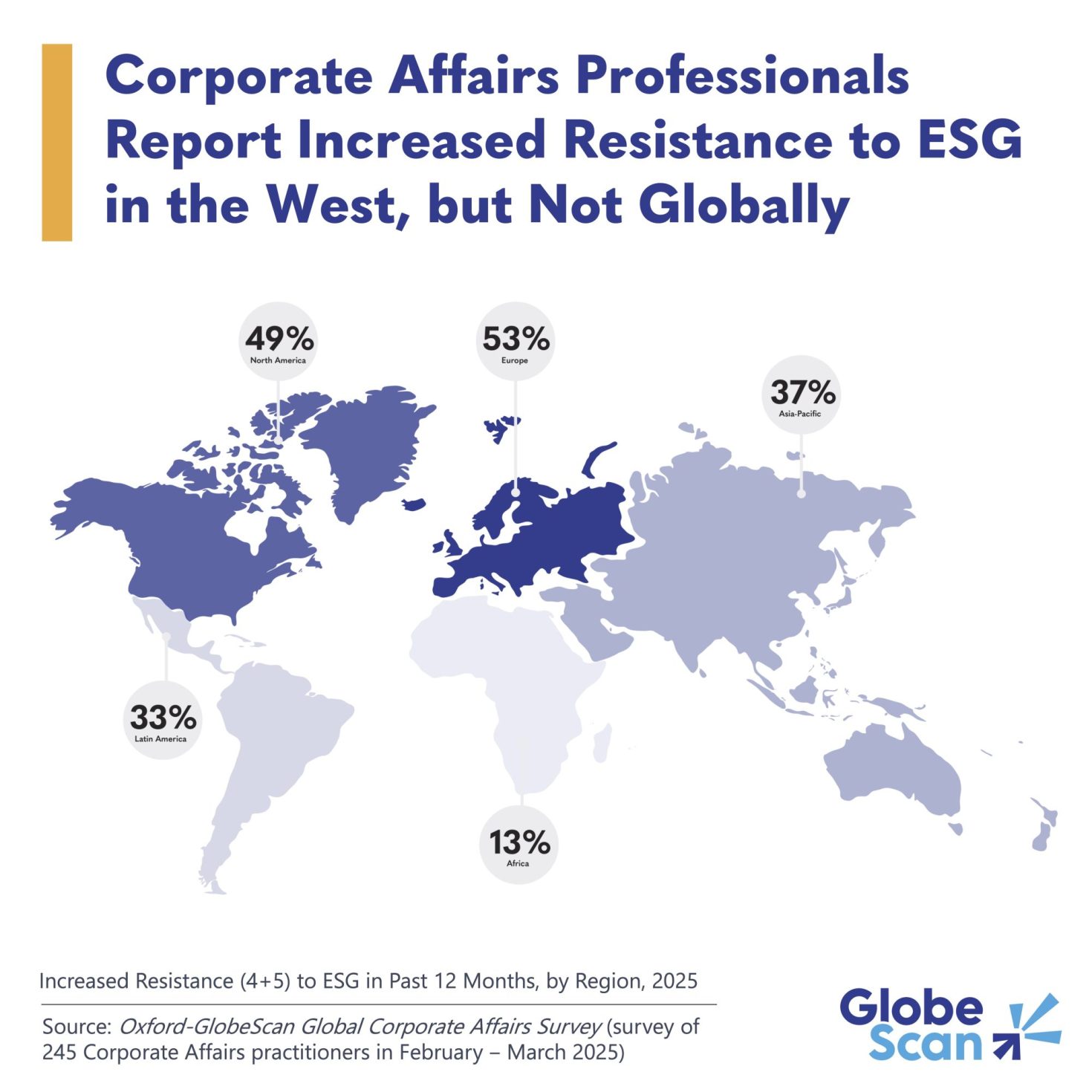
FDA's Prasad details decision to pause chikungunya vaccine in seniors
The FDA and CDC's decision last month to pause administering Valneva's live-attenuated chikungunya vaccine in patients 60 and older was meant to provide more time to examine a potential causal relationship between the vaccine and ...