FDA rejects Stealth's rare disease drug, but offers potential for accelerated approval
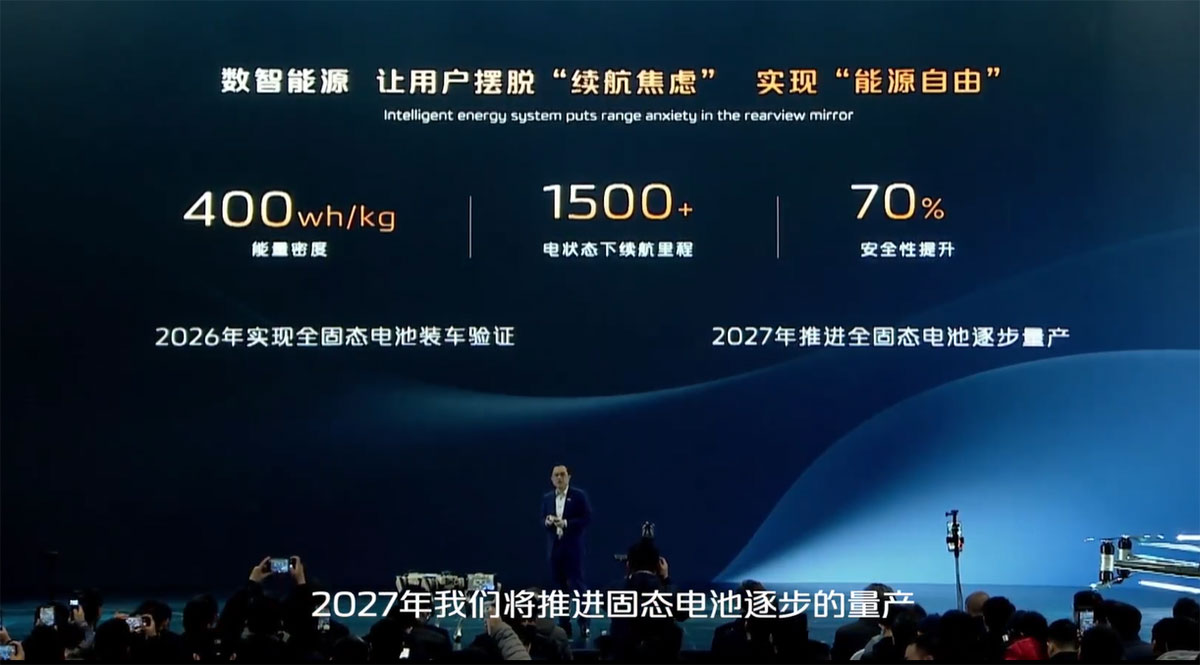
After a slight delay, the FDA rejected Stealth BioTherapeutics' Barth syndrome drug application for elamipretide, the company said early Thursday, but will consider a resubmission via the accelerated approval pathway. The complete response letter included ...