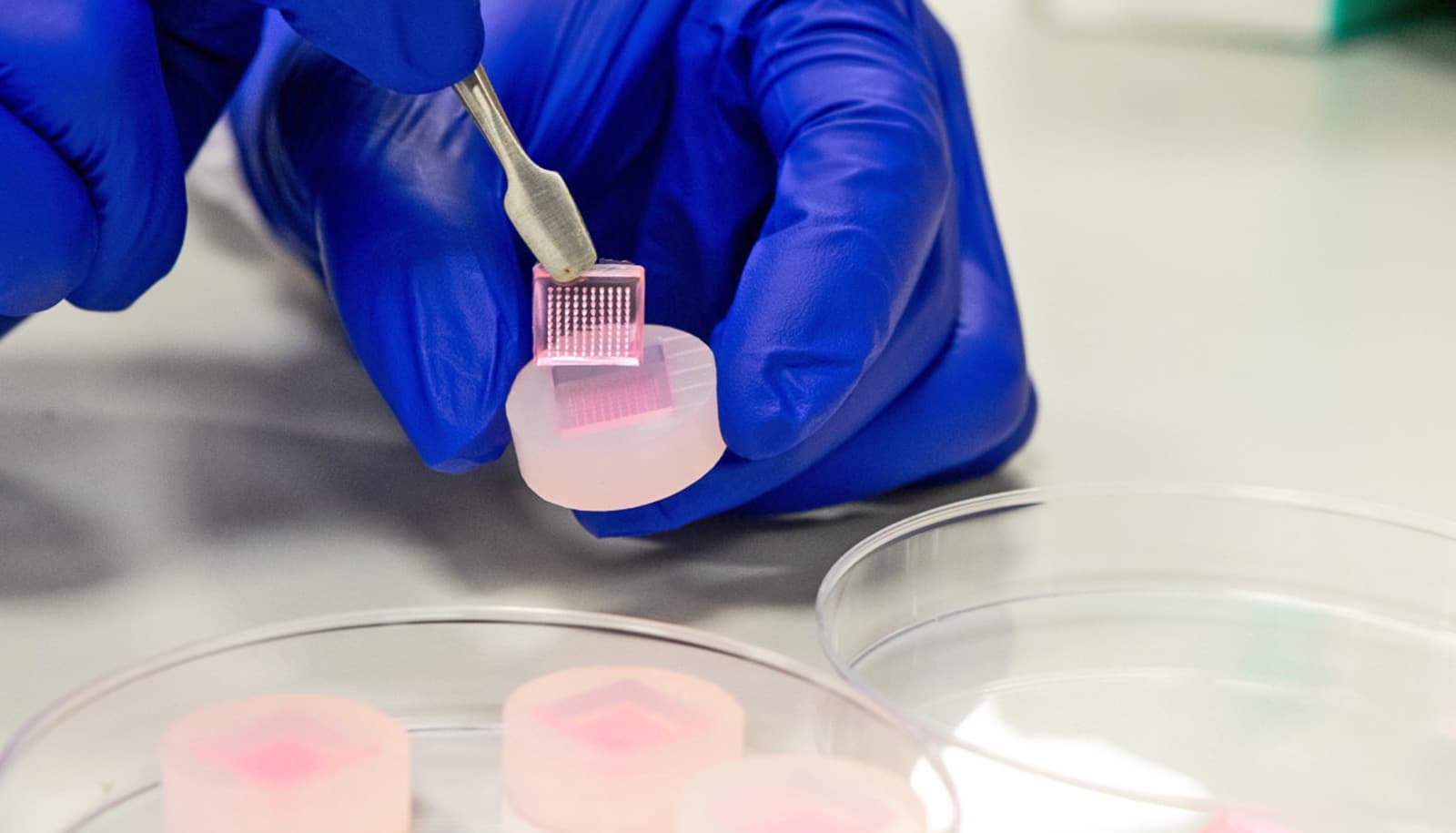
FDA Phase Out of Animal Test Requirements for Drugs Begins With Biologics
The FDA laid out a three-year roadmap to phase out animal toxicity testing in drug research. In addition to organoids and lab-on-a-chip tests, the agency is encouraging drug companies to use computer simulations and artificial intelligence to predict drug behavior. The post FDA Phase Out of Animal Test Requirements for Drugs Begins With Biologics appeared first on MedCity News.

The FDA laid out a three-year roadmap to phase out animal toxicity testing in drug research. In addition to organoids and lab-on-a-chip tests, the agency is encouraging drug companies to use computer simulations and artificial intelligence to predict drug behavior.
The post FDA Phase Out of Animal Test Requirements for Drugs Begins With Biologics appeared first on MedCity News.
















































































!['The Dream is [still] Alive': First IMAX film shot in space at 40 years](https://cdn.mos.cms.futurecdn.net/ii3bo2jM3f9hmrYbo3p7pF.jpg?#)



































![The breaking news round-up: Decagear launches today, Pimax announces new headsets, and more! [APRIL FOOL’S]](https://i0.wp.com/skarredghost.com/wp-content/uploads/2025/03/lawk_glasses_handson.jpg?fit=1366%2C1025&ssl=1)