FDA lifts hold on Entrada's Duchenne drug after two years
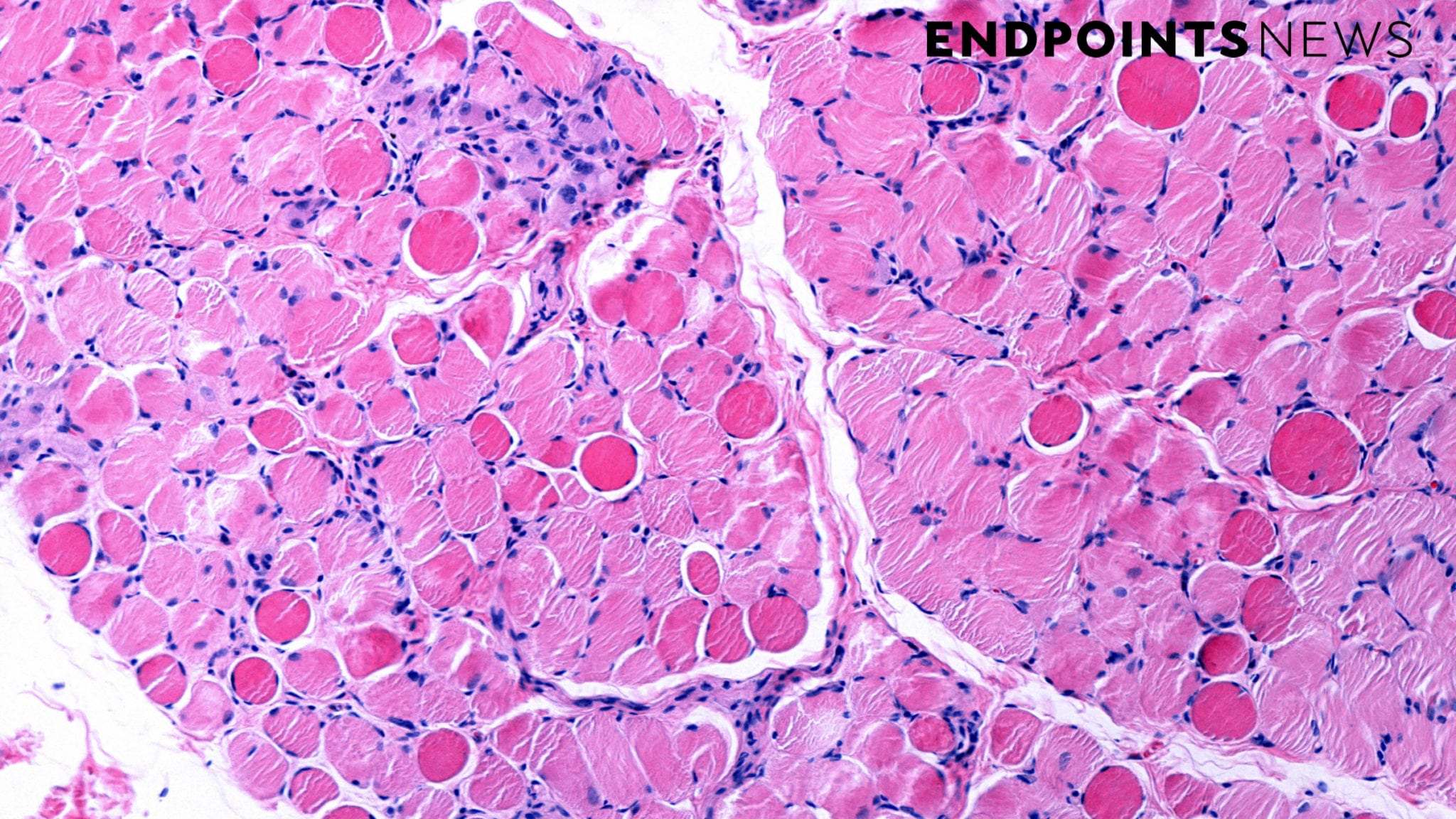
Entrada Therapeutics announced Monday that the FDA lifted the clinical hold on its Duchenne muscular dystrophy drug, and it plans to start a Phase 1b study in the US in 2026. The biotech
Apr 9, 2025 0
Apr 9, 2025 0
Apr 9, 2025 0
Apr 9, 2025 0
Or register with email

Feb 13, 2025 0
Feb 9, 2025 0
Feb 9, 2025 0
Feb 9, 2025 0
Feb 19, 2025 0
This site uses cookies. By continuing to browse the site you are agreeing to our use of cookies.
