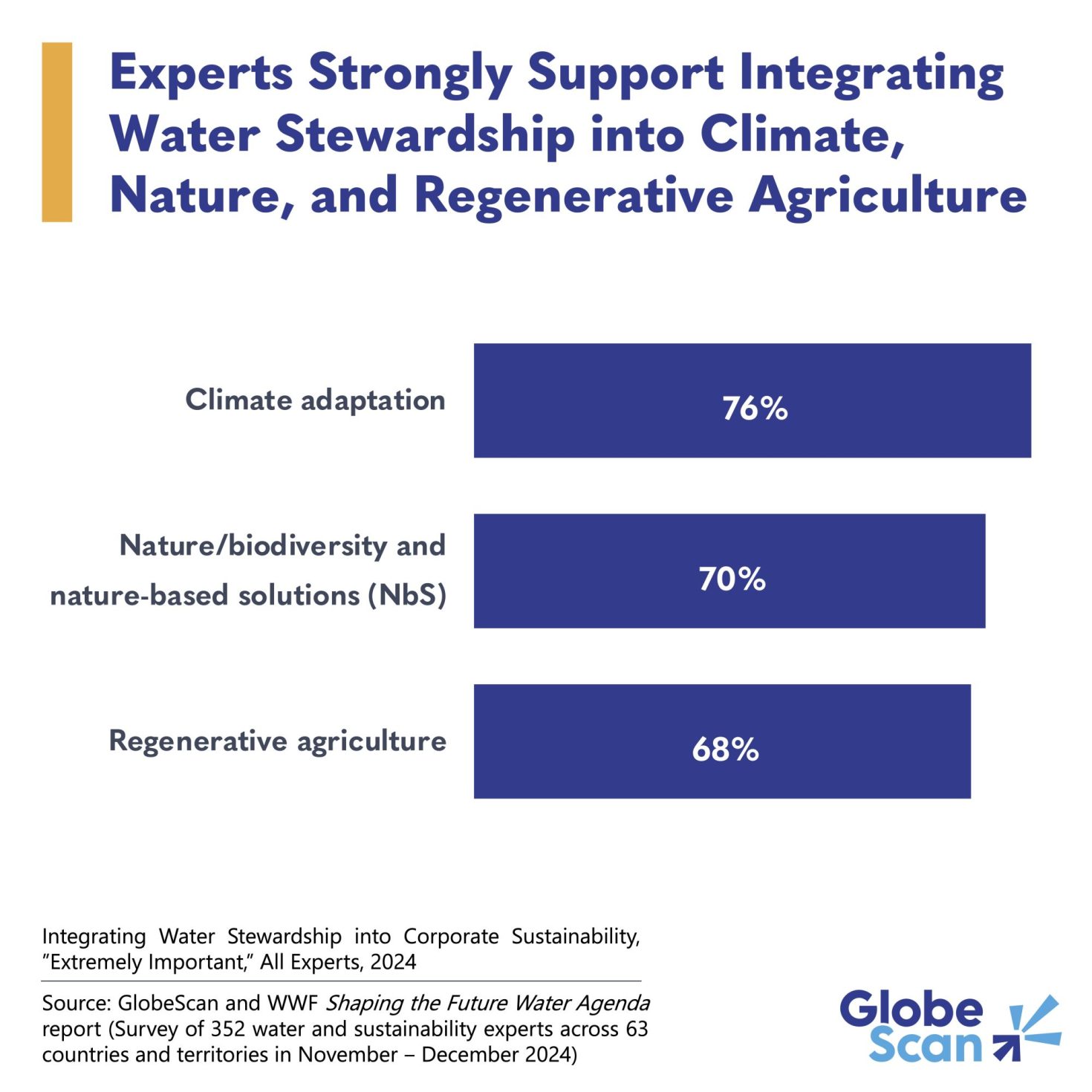
FDA critiques 'misleading' Taiho promotion of bile duct cancer drug
The FDA is taking issue with a promotional website from Taiho Oncology, alleging it misleadingly describes the benefits of its bile duct cancer drug Lytgobi, which won accelerated approval in 2022. The





































































































.jpg?#)




































