Chipiron Secures $17M to Transform MRI Access with Portable Scanner
What You Should Know: – French deeptech startup Chipiron today announced it has raised $17M in a Series A funding round to finalize the development of its compact, affordable MRI system. – Led by Blast, with participation from the EIC Fund and iXcore, and supported by public funding initiatives including France2030 and the EIC Accelerator, ... Read More


What You Should Know:
– French deeptech startup Chipiron today announced it has raised $17M in a Series A funding round to finalize the development of its compact, affordable MRI system.
– Led by Blast, with participation from the EIC Fund and iXcore, and supported by public funding initiatives including France2030 and the EIC Accelerator, the investment aims to make highly accurate MRI technology accessible worldwide.
Tackling MRI Accessibility Challenges
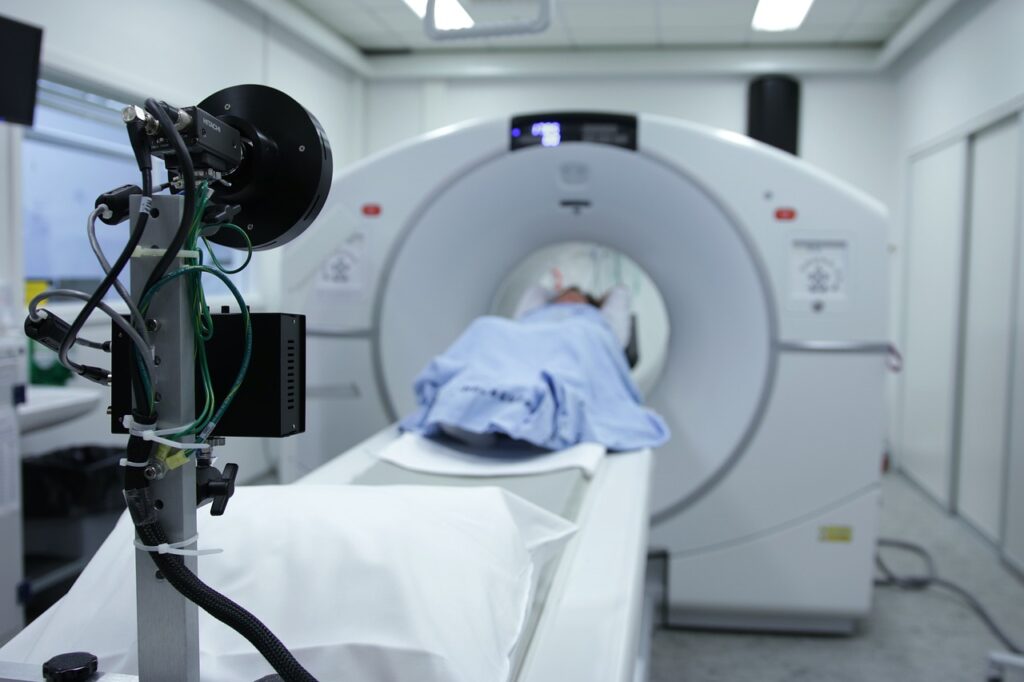
Magnetic Resonance Imaging (MRI) is widely regarded as the most precise medical imaging modality available, yet its use remains limited. Traditional MRI systems are large, extremely expensive, and require complex hospital infrastructure, restricting their deployment primarily to major hospitals. This lack of accessibility contributes to disparities in diagnostic capabilities and patient care. The global MRI market, estimated at $10B, has the potential to double if the technology becomes more widely available.
Founded in 2020 by CEO Evan Kervella and CSO Dimitri Labat, Chipiron aims to make MRI as accessible as an X-ray. The company is developing a next-generation, ultra-low-field MRI machine that is designed to be as reliable as current models but significantly more compact, mobile, and affordable – potentially 10 times less expensive than current standards. This breakthrough design opens up possibilities for deployment in facilities previously excluded from MRI use, such as local care centers, private clinics, and mobile units. Furthermore, the smaller size makes MRI scans feasible for patients often excluded from traditional machines, including those with pacemakers or implants, claustrophobic individuals, obese or elderly patients, and restless children.
Expansion Plans
The company plans to produce its first clinical prototypes by the end of 2025 and commence investigational trials in hospital settings starting in 2026. Looking ahead three to five years, Chipiron has clear objectives: achieve an initial clinical application, secure the first FDA (US) and CE (Europe) regulatory authorizations, and deploy 100 commercial devices. The company plans a significant focus on expanding into the extensive US healthcare market, known for its adoption of innovative solutions, with the ultimate goal of establishing a new global standard in accessible medical imaging.