FDA Just Made Key Some Key Regulatory Changes to Make Cancer Cell Therapies More Accessible
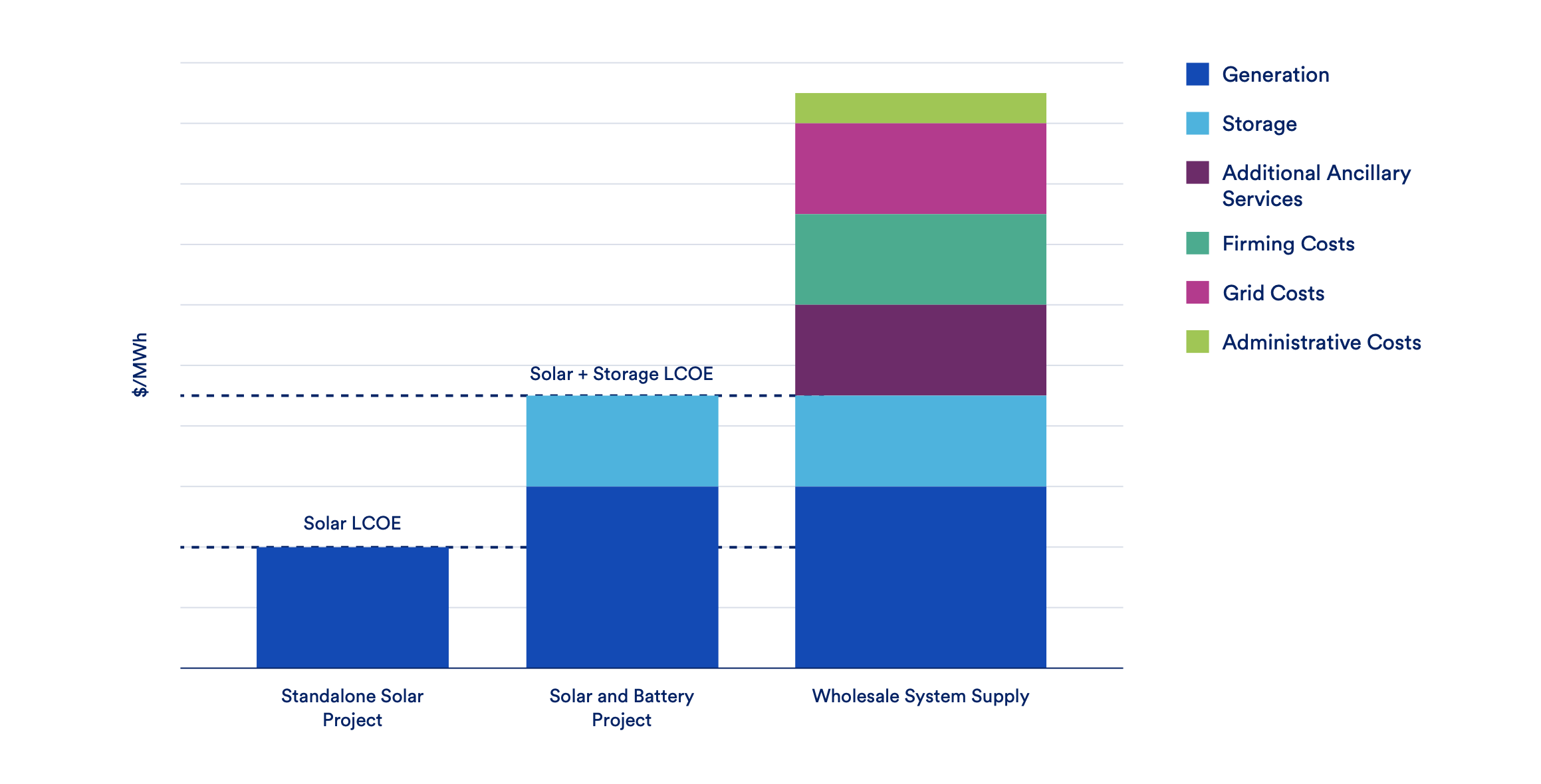
The FDA has eliminated a safety monitoring requirement and eased certain restrictions that had been in place since CAR T-therapies first reached the market in 2017. The agency and financial analysts say the changes should improve patient access to these personalized cancer treatments. The post FDA Just Made Key Some Key Regulatory Changes to Make Cancer Cell Therapies More Accessible appeared first on MedCity News.
The FDA has eliminated a safety monitoring requirement and eased certain restrictions that had been in place since CAR T-therapies first reached the market in 2017. The agency and financial analysts say the changes should improve patient access to these personalized cancer treatments.
The post FDA Just Made Key Some Key Regulatory Changes to Make Cancer Cell Therapies More Accessible appeared first on MedCity News.